Study designs Basic PK concepts and measurements77 Basic PK concepts and measurements77
Area under the curve (AUC)
The overall amount of drug in the bloodstream after a dose
Generating an AUC requires collecting many blood samples (usually every one or two hours) right after a person takes a dose up until the next dose is due
Bioavailability78
Measures of the amount of intact drug that reaches the systemic circulation
Provides information about the dose/dosage regimen and performance of different formulations
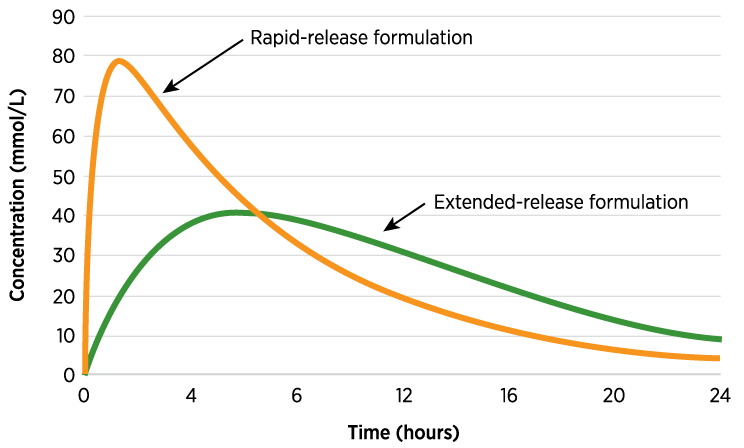
Bioequivalence78
Formulations containing the same dose of the same chemical entity, generally in the same dosage form, and that are intended to be interchangeable are deemed bioequivalent
This information may be useful for evaluating formulation changes (tablet vs. capsule) and comparing generic and branded drugs
Drugs that are bioequivalent are not expected to differ in clinical and adverse events
Cmax
(maximum concentration)
The highest concentration of drug in the blood that is measured after a dose
Cmax usually happens within a few hours after the dose is taken
The time that Cmax happens is referred to as Tmax
Cmin or trough
(minimum concentration)
The lowest concentration of the drug in the blood that is measured after a dose
It happens right before a patient takes the next usual dose
Half-life (t½)
The amount of time it takes for the drug concentration in the blood to decline by half
t½ is one of the most important PK measurements for how often a drug has to be dosed
Jump to:
Overview Parallel double-blind placebo controlled Crossover Laboratory school protocol Adult workplace environment Pharmacokinetic studiesBasic PK measurements
